Background
Somatic mutations in the protein phosphatase non-receptor type 11 ( PTPN11) gene occur frequently in children with juvenile myelomonocytic leukemia and less commonly in adults with AML, being present in less than 5% of reported cases. Uncertainty exists regarding the prognostic impact of PTPN11 mutations in AML, largely owing to their observed rarity. Some retrospective studies have identified PTPN11 mutations as deleterious prognostic markers in AML, though others have noted that their negative impact is mitigated by concurrent NPM1 mutations, which frequently co-occur. We analyzed the outcomes of patients at our institution with AML and PTPN11 mutations in order to add to the literature regarding the relevance of this mutation.
Methods
We conducted a retrospective case series of patients at a single-center, tertiary-care institution who were diagnosed with AML between January 1 st, 2020 and June 1 st, 2023. Inclusion criteria included: 18 years of age or older, primary follow-up at the study institution, documented diagnosis of AML and identification of a PTPN11 mutation by NGS. Outcomes of interest included cytogenetic and molecular profiles, response to induction or salvage therapy, progression-free survival (PFS) and overall survival (OS).
Results
Twenty-two (12 men and 10 women) patients with PTPN11 mutant AML were identified. The median age at diagnosis was 60 years (range, 31-89 years). Nineteen patients had a PTPN11 mutation identified at diagnosis and 3 had a PTPN11 mutation appear at the time of relapse.
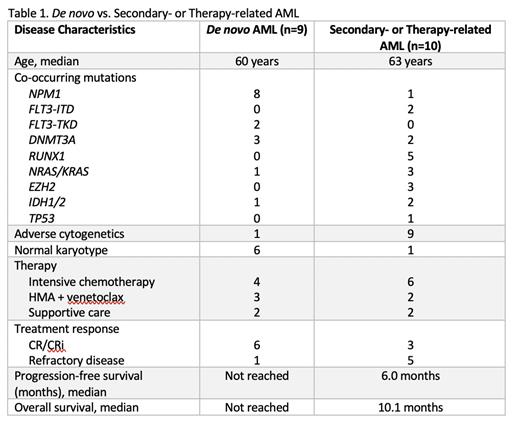
Nine patients with de novo AML had PTPN11 mutations identified at diagnosis. Six were female and the average age at diagnosis was 60 years (range, 44-89 years). The most common co-occurring mutations were NPM1 (8 patients), DNMT3A (3 patients), TET2 (2 patients) and FLT3-TKD (2 patients). Cytogenetics were normal or non-adverse in 8 patients, and complex/adverse-risk in 1 patient. Seven patients received treatment - 4 with intensive chemotherapy and 3 with a hypomethylating agent (HMA) + venetoclax. Treatment response was CR/CRi in 6 patients (85.7%). At a median follow-up of 195 days, median PFS and OS were not reached. Two patients relapsed at 76 and 139 days after response. No patients received an allogeneic transplant in first remission.
Ten patients with therapy-related or secondary-AML (t/s-AML) were identified, all with PTPN11 mutations noted at the time of AML diagnosis. Two patients had preceding MDS and 3 had preceding MDS/MPN. Median age was 63 years (range, 31-70 years). The most common co-occurring mutations were RUNX1 (5 patients), FLT3-ITD (2 patients) and NRAS/KRAS (3 patients). Chromosome 7 abnormalities were present in 4 of 5 patients with RUNX1 mutations. Cytogenetics were complex or adverse-risk in 9 patients. Eight patients received treatment - 6 with intensive chemotherapy and 2 with HMA + venetoclax. Treatment response was CR/CRi in 3 patients (37.5%). Median PFS was 183 days and median OS in treated patients was 307 days. The only patient with an ongoing response at the time of data cut-off received an allogeneic transplantation in CR1.
Three patients with AML had emergence of a PTPN11 mutation at the time of relapse, at 245, 343 and 2262 days after initial diagnosis. PTPN11 was the only new mutation identified in 2 patients, and emerged in conjunction with an NRAS and KDM6A mutation in 1 patient. One patient received treatment, attaining a CRi after HMA + venetoclax, but relapsed 112 days later. The other 2 patients passed at 10 and 21 days after relapse.
Conclusions
PTPN11 mutations were relatively rare, occurring in only 22 patients with AML during a 3.5-year time span. Stark differences were noted in patients with de novo AML versus t/s-AML and PTPN11 mutations. De novo AML patients frequently demonstrated NPM1 mutations and a normal karyotype, while t/s-AML patients frequently had RUNX1 and FLT3 mutations, as well as adverse cytogenetics. Although the impact of PTPN11 mutations in AML may be influenced by concurrent NPM1 mutations or cytogenetic characteristics, the context of AML may ultimately have the largest impact.
Disclosures
Baratam:ONO Pharma: Consultancy; Protagnoist Pharma: Consultancy; Rigel: Consultancy; KITE: Consultancy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal